In Silico Study of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) Sequence in Citrus Associated with Huanglongbing Resistance
DOI:
https://doi.org/10.70158/buitenzorg.v2i2.25Abstract
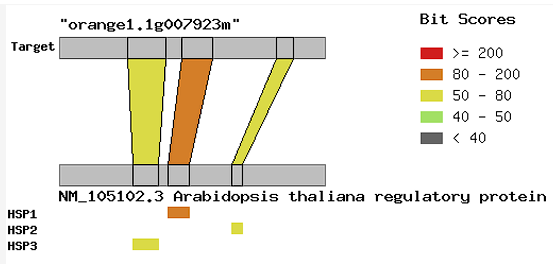
The evaluation of citrus resistance to Huanglongbing (HLB) disease is still challenging due to the incapability of the bacteria to be cultured purely in artificial medium, the complexity of inoculation methods, and the long duration required for phenotypic observation. Thus, the use of molecular markers is one of the alternatives to solve this problem. The focuses of this study were to perform in silico analysis of the nucleotide variations in NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) gene sequence among several citrus genotypes whose resistance information to HLB have been known previously and to employ phylogenetic analysis among them. The NPR1 gene sequences from 20 genotypes which consisted of 14 citrus and six relative genotypes were collected in silico from Citrus Genome Database and analyzed using multiple sequence alignment program. A total of six interesting SNPs that could distinguish between susceptible and resistant citrus genotypes were detected in this study. As many as five SNPs were non-synonymous, while only one synonymous SNP that did not cause the amino acid change was identified in this study. The phylogenetic analysis also revealed the separation between susceptible and tolerant/resistant citrus genotypes in two main clusters. The SNPs found in this study are expected to be useful for designing new functional markers as a selection tool in future studies.
Keywords: in silico, molecular marker, phylogenetic analysis, selection tool, SNP
Downloads
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Alves, M. N., Lopes, S. A., Raiol-Junior, L. L., Wulff, N. A., Girardi, E. A., Ollitrault, P., & Peña, L. (2021). Resistance to ‘Candidatus Liberibacter asiaticus,’ the Huanglongbing associated bacterium, in sexually and/or graft-compatible citrus relatives. Frontiers in Plant Science, 11, 617664. https://doi.org/10.3389/ fpls.2020.617664
Bové, J. M. (2006). Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. Journal of Plant Pathology, 88(1), 7–37. https://doi.org/10.1371/journal.pone.0111032
Cao, J., Cheng, C., Yang, J., & Wang, Q. (2015). Pathogen infection drives patterns of nutrient resorption in citrus plants. Scientific Reports, 5(1), 14675. https://doi.org/10.1038/srep14675
Chen, H., McCollum, G., Baldwin, E., & Bai, J. (2016). Impacts of Huanglongbing symptom severity on fruit detachment force and mechanical properties of sweet oranges (Citrus sinensis). HortScience, 51(4), 356–361. https://doi.org/10.21273/HORTSCI.51.4.356
Cui, X., Zhang, J., Liu, Y., Luo, X., Deng, X., Zhang, S., & Xu, M. (2022). Comparison of different grafting methods on the effect of 'Candidatus Liberibacter asiaticus' transmission. Fruit Research, 2, 15. http://doi.org/10.48130/FruRes-2023-0002
Davis, M. J., Mondal, S. N., Chen, H., Rogers, M. E., & Brlansky, R. H. (2008). Co-cultivation of ‘Candidatus Liberibacter asiaticus’ with Actinobacteria from citrus with Huanglongbing. Plant Disease, 92(11), 1547–1550. https://doi.org/10.1094/ PDIS-92-11-1547
De Mori, G., & Cipriani, G. (2023). Marker-assisted selection in breeding for fruit trait improvement: a review. International Journal of Molecular Sciences, 24(10), 8984. https://doi.org/10.3390/ijms24108984
Deng, X., Zhou, G., Li, H., Chen, J., & Civerolo, E. L. (2007). Detection of Candidatus Liberibacter asiaticus from wampee (Clausena lansium Skeels) by nested PCR. Plant Health Progress, 8(1). https://doi.org/10.1094/PHP-2007-0419-01-BR
Ding, F., Wang, G., Yi, G., Zhong, Y., Zeng, J., & Zhou, B. (2005). Infection of wampee and lemon by the citrus Huanglongbing pathogen (Candidatus Liberibacter asiaticus) in China. Journal of Plant Pathology, 87(3), 207–212. https://www.jstor.org/stable/41998240
Dong, L., Chen, S., Shang, L., Du, M., Mo, K., Pang, S., Zheng, L., Xu, L., Lei, T., He, Y., & Zou, X. (2024). Overexpressing CsSABP2 enhances tolerance to Huanglongbing and citrus canker in C. sinensis. Frontiers in Plant Science, 15, 1472155. https://doi.org/10.3389/fpls.2024.1472155
Duan, Y., Zhou, L., Hall, D. G., Li, W., Doddapaneni, H., Lin, H., Liu, L., Vahling, C. M., Gabriel, D. W., Williams, K. P., Dickerman, A., Sun, Y., & Gottwald, T. (2009). Complete genome sequence of citrus Huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Molecular Plant-Microbe Interactions, 22(8), 1011–1020. https://doi.org/10.1094/MPMI-22-8-1011
Dutt, M., Barthe, G., Irey, M., & Grosser, J. (2015). Transgenic citrus expressing an Arabidopsis NPR1 gene exhibit enhanced resistance against Huanglongbing (HLB; citrus greening). PLoS ONE, 10(9), e0137134. https://doi.org/10.1371/journal.pone.0137134
Folimonova, S. Y., Robertson, C. J., Garnsey, S. M., Gowda, S., & Dawson, W. O. (2009). Examination of the responses of different genotypes of citrus to Huanglongbing (citrus greening) under different conditions. Phytopathology, 99(12), 1346–1354. https://doi.org/10.1094/PHYTO-99-12-1346
Grafton-Cardwell, E. E., Stelinski, L. L., & Stansly, P. A. (2013). Biology and management of Asian citrus psyllid, vector of the Huanglongbing pathogens. Annual Review of Entomology, 58, 413–432. https://doi.org/10.1146/annurev-ento-120811-153542
Granato, L. M., Galdeano, D. M., D’Alessandre, N. D. R., Breton, M. C., & Machado, M. A. (2019). Callose synthase family genes plays an important role in the citrus defense response to Candidatus Liberibacter asiaticus. European Journal of Plant Pathology, 155, 25–38. https://doi.org/10.1007/s10658-019-01747-6
Hall, D. G., Hentz, M. G., & Stover, E. (2012). Field survey of Asian citrus psyllid (Hemiptera: Liviidae) infestations associated with six cultivars of Poncirus trifoliata (Rutaceae). Florida Entomology, 100(3), 667–668. https://journals.flvc.org/flaent/article/view/90823/100959
Hijaz, F., Nehela, Y., & Killiny, N. (2016). Possible role of plant volatiles in tolerance against Huanglongbing in citrus. Plant Signaling and Behavior, 11(3), e1138193. https://doi.org/10.4161/psb.25677
Jagoueix, S., Bové, J. M., & Garnier, M. (1994). The phloem-limited bacterium of greening disease of citrus is a member of the α subdivision of the Proteobacteria. International Journal of Systematic Bacteriology, 44(3), 379–386. https://doi.org/10.1099/00207713-44-3-379
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P., & Drummond, A. (2012). Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kosmiatin, M., Martasari, C., Akhdiya, A., & Husni, A. (2020). In vitro selection to increase Huanglongbing tolerance of citrus derived from in vitro breeding. IOP Conference Series: Earth and Environmental Science, 457(1), 012080. https://doi.org/10.1088/1755-1315/457/1/012080
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549. https://doi.org/10.1093/molbev/msy096
Li, J., Brader, G., & Palva, E. T. (2004). The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. The Plant Cell, 16(2), 319–331. https://doi.org/10.1105/tpc.016980
Li, J., Trivedi, P., & Wang, N. (2016). Field evaluation of plant defense inducers for the control of citrus Huanglongbing. Phytopathology, 106(1), 37–46. https://doi.org/10.1094/PHYTO-08-15-0196-R
Liao, H., Liu, F., Wang, X., Huang, H., Huang, Q., Wang, N., & Wei, C. (2025). Comparative transcriptome analysis of susceptible and resistant rutaceae plants to Huanglongbing. Agronomy, 15, 1218. https://doi.org/10.3390/agronomy15051218
Lin, H., Chen, C., Doddapaneni, H., Duan, Y., Civerolo, E. L., Bai, X., & Zhao, X. (2010). A new diagnostic system for ultra-sensitive and specific detection and quantification of Candidatus Liberibacter asiaticus, the bacterium associated with citrus Huanglongbing. Journal of Microbiological Methods, 81, 17–25. https://doi.org/110.1016/j.mimet.2010.01.014
Lopes, S. A., & Cifuentes-Arenas, J. C. (2021). Protocol for successful transmission of ‘Candidatus Liberibacter asiaticus’ from citrus to citrus using Diaphorina citri. Phytopathology, 111, 2367–2374 https://doi.org/10.1094/PHYTO-02-21-0076-R
Mafra, V., Kubo, K. S., Alves-Ferreira, M., Ribeiro-Alves, M., Stuart, R. M., Boava, L. P., Rodrigues, C. M., & Machado, M. A. (2012). Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE, 7(2), e31263. https://doi.org/10.1371/journal.pone.0031263
Muñoz-Fambuena, N., Nicolás-Almansa, M., Martínez-Fuentes, A., Reig, C., Iglesias, D. J., Primo-Millo, E., Mesejo, C., & Agustí, M. (2019). Genetic inhibition of flowering differs between juvenile and adult citrus trees. Annals of Botany, 123(3), 483–490. https://doi.org/10.1093/aob/mcy179
Nehela, Y., & Killiny, N. (2020). Revisiting the complex pathosystem of Huanglongbing: deciphering the role of citrus metabolites in symptom development. Metabolites, 10(10), 409. https://doi.org/10.3390/metabo10100409
Noflindawati, Anwar, A., Sutanto, A., & Yusniwati (2021). Optimization of annealing cycle and temperature SNAP T12 primer distinguishing markers for male, female and hermaphrodite plants in papaya (Carica papaya L.). IOP Conference Series: Earth and Environmental Science, 715, 012040. https://doi.org/10.1088/ 1755-1315/715/1/012040
Nugroho, K., Purwito, A., Sukma, D., Kosmiatin, M., Santoso, T. J., Husni, A., Martasari, C., & Lestari, P. (2025a). Molecular diversity of citrus genotypes using callose synthase 7 gene markers linked to Huanglongbing resistance. Jurnal Agronomi Indonesia, 53(2), 212–223. https://dx.doi.org/10.24831/jai.v53i2.64952
Nugroho, K., Purwito, A., Sukma, D., Kosmiatin, M., Santoso, T. J., Reflinur, & Mastur (2025b). Nucleotide variations of WRKY70 gene sequences related to Huanglongbing resistance in citrus. Jurnal Biologi Tropis, 25(4), 5731–5742. http://doi.org/10.29303/jbt.v25i4.10092
Pandey, S. S., Hendrich, C., Andrade, M. O., & Wang, N. (2022). Candidatus Liberibacter: from movement, host responses, to symptom development of citrus Huanglongbing. Phytopathology, 112, 55–68. https://doi.org/10.1094/PHYTO-08 -21-0354-FI
Pang, Z., Zhang, L., Coaker, G., Ma, W., He, S. Y., & Wang, N. (2020). Citrus CsACD2 is a target of Candidatus Liberibacter asiaticus in Huanglongbing disease. Plant Physiology, 184(2), 792–805. https://doi.org/10.1104/pp.20.00348
Parker, J. K., Wisotsky, S. R., Johnson, E. G., Hijaz, F. M., Killiny, N., Hilf, M. E., & La Fuente, L. D. (2014). Viability of ‘Candidatus Liberibacter asiaticus’ prolonged by addition of citrus juice to culture medium. Phytopathology, 104(1), 15–26. http://dx.doi.org/10.1094/PHYTO-05-13-0119-R
Pesik, A., Efendi, D., Novarianto, H., Dinarti, D., & Sudarsono, S. (2017). Development of SNAP markers based on nucleotide variability of WRKY genes in coconut and their validation using multiplex PCR. Biodiversitas, Journal of Biological Diversity, 18(2), 465–475. https://doi.org/10.13057/biodiv/d180204
Prasetyaningrum, P., Purwantoro, A., & Subandiyah, S. (2012). Genetics diversity analysis of six difference varieties of pomelo (Citrus maxima [Burm.] Merr.) for resistance to Huanglongbing using RAPD markers. Vegetalika, 1(2), 78–85. https://doi.org/10.22146/veg.1521
Prihatini, R., Dinarti D., Sutanto, A., & Sudarsono (2022). Development of hermaphrodite salacca (Salacca zalacca) SNAP marker: a novel conservation tool. IOP Conference Series: Earth and Environmental Science, 1105, 012030. https://doi.org/10.1088/1755-1315/1105/1/012030
Puttamuk, T., Zhang, S., Duan, Y., Jantasorn, A., & Thaveechai, N. (2014). Effect of chemical treatments on ‘Candidatus Liberibacter asiaticus’ infected pomelo (Citrus maxima). Crop Protection, 65, 114–121. https://doi.org/10.1016/j.cropro.2014.07.018
Qiu, W., Soares, J., Pang, Z., Huang, Y., Sun, Z., Wang, N., Grosser, J., & Dutt, M. (2020). Potential mechanisms of AtNPR1 mediated resistance against Huanglongbing (HLB) in citrus. International Journal of Molecular Sciences, 21(6), 2009. https://doi.org/10.3390/ijms21062009
Ramadugu, C., Keremane, M. L., Halbert, S. E., Duan, Y. P., Roose, M. L., Stover, E., & Lee, R. F. (2016). Long-term field evaluation reveals Huanglongbing resistance in citrus relatives. Plant Disease, 100(9), 1858–1869. https://doi.org/10.1094/PDIS-03-16-0271-RE
Ramekar, S., Mahmoud, L. M., Deol, J. K., Welker, S., & Dutt, M. (2025). Exploring the biochemical and molecular mechanisms that contribute to Huanglongbing (HLB) tolerance in Citrus australis hybrids. BMC Genomics, 26, 761. https://doi.org/10.1186/s12864-025-11942-x
Sechler, A., Schuenzel, E. L., Cooke, P., Donnua, S., Thaveechai, N., Postnikova, E., Stone, A. L., Schneider, W. L., Damsteegt, V. D., & Schaad, N. W. (2009). Cultivation of ‘Candidatus Liberibacter asiaticus’, ‘Ca. L. africanus’, and ‘Ca. L. americanus’ associated with Huanglongbing. Phytopathology, 99(5), 480–486. https://doi.org/10.1094/PHYTO-99-5-0480
Shokrollah, H., Abdullah, T. L., Sijam, K., Abdullah, S. N. A., & Abdullah, N. A. P. (2009). Differential reaction of citrus species in Malaysia to Huanglongbing (HLB) disease using grafting method. American Journal of Agricultural and Biological Science, 4(1), 32–38. https://doi.org/10.3844/ajabssp.2009.32.38
Sievers, F., & Higgins, D. G. (2014). Clustal Omega. Current Protocols in Bioinformatics, 48(1), 3–13. https://doi.org/10.1002/0471250953.bi0313s48
Sukma, D., Elina, J., Raynalta, E., Aisyah, S. I., Aziz, S. A., Sudarsono, S., & Chan, M. T. (2021). Analysis of the genetic diversity of Phalaenopsis orchids with single nucleotide polymorphisms and SNAP markers derived from the Pto gene. SABRAO Journal of Breeding and Genetics, 53(4), 620–631. https://doi.org/10.54910/sabrao2021.53.4.6
Tarigan, R., Maharijaya, A., & Izzah, N. K. (2021). SNAP markers derived from catalase-1 gene sequence used for black pod disease resistance in cacao (Theobroma cacao L.). SABRAO Journal of Breeding and Genetics, 53(3), 510–526.
Terryana, R. T., Rijzaani, H., Priyatno, T. P., Manzila, I., & Lestari, P. (2020). Construction of DNA fingerprint for chili pepper varieties using SNAP markers. IOP Conference Series: Earth and Environmental Science, 482(1), 012038. https://doi.org/ 10.1088/1755-1315/482/1/012038
Tipu, M. M. H., Masud, M. M., Jahan, R., Baroi, A., & Hoque, A. K. M. A. (2021). Identification of citrus greening based on visual symptoms: a grower's diagnostic toolkit. Heliyon, 7(11), e08387. https://doi.org/10.1016/j.heliyon.2021.e08387
Tsai, C. H., Su, H. J., Liao, Y. C., & Hung, T. H. (2006). First report of the causal agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) infecting kumquat in Taiwan. Plant Disease, 90, 1360. https://doi.org/10.1094/PD-90-1360C
Tsai, C. H., Hung, T. H., & Su, H. J. (2008). Strain identification and distribution of citrus Huanglongbing bacteria in Taiwan. Botanical Studies, 49, 49–56.
Wang, X. L., Hayat, F., Li, J., Peng, Y., Li, D. S., Ma, X. Y., Ahmed, N., Tu, P. F., Chen, J. Z., Xu, M. Q., & Gong, L. (2025). Citrus rootstock selection for enhanced Huanglongbing resistance: a strategic disease management paradigm. Applied Ecology and Environmental Research, 23(2), 3107–3123. http://dx.doi.org/10.15666/aeer/2302_31073123
Weber, K. C., Mahmoud, L. M., Stanton, D., Welker, S., Qiu, W., Grosser, J. W., Levy, M., & Dutt, M. (2022). Insights into the mechanism of Huanglongbing tolerance in the Australian finger lime (Citrus australasica). Frontiers in Plant Science, 13, 1019295. https://doi.org/10.3389/fpls.2022.1019295
Widyaningsih, S. U., Hidayah S. N., Joko, T., & Subandiyah, S. (2019). Plant response and Huanglongbing disease development against heat treatments on ‘Siam Purworejo’ (Citrus nobilis [Lour]) and ‘Nambangan’ (C. maxima [Burm.] Merr.) under field condition. Archives of Phytopathology and Plant Protection, 52(3–4), 259–276. https://doi.org/10.1080/03235408.2018.1544193
Wu, H., Hu, Y., Fu, S., Zhou, C., & Wang, X. (2020). Coordination of multiple regulation pathways contributes to the tolerance of a wild citrus species (Citrus ichangensis ‘2586’) against Huanglongbing. Physiological and Molecular Plant Pathology, 109, 101457. https://doi.org/10.1016/j.pmpp.2019.101457
Wu, Q., Moniruzzaman, M., Yan, H., Lv, Y., Jiang, B., Jiang, N., & Zhong, Y. (2021). The CsNPR1 gene expression modulation in citrus and understanding the defense mechanism against Huanglongbing by screening CsNPR1-interacting proteins. Scientia Horticulturae, 288, 110375. https://doi.org/10.1016/j.scienta.2021.110375
Xu, Y., Jia, H., Tan, C., Wu, X., Deng, X., & Xu, Q. (2022). Apomixis: genetic basis and controlling genes. Horticulture Research, 9, uhac150. https://doi.org/10.1093/hr/uhac150
Yu, S. S., Zhu, A. N., Song, W. W., & Yan, W. (2022). Molecular identification and characterization of two groups of phytoplasma and Candidatus Liberibacter asiaticus in single or mixed infection of Citrus maxima on Hainan Island of China. Biology, 11(6), 869. https://doi.org/10.3390/biology11060869
Zeng, Z., & Bromberg, Y. (2019). Predicting functional effects of synonymous variants: a systematic review and perspectives. Frontiers in Genetics, 10, 914. https://doi.org/10.3389/fgene.2019.00914
Zhang, J., Sun, L., Wang, Y., Li, B., Li, X., Ye, Z., & Zhang, J. (2024). A calcium-dependent protein kinase regulates the defense response in Citrus sinensis. Molecular Plant-Microbe Interactions, 37(5), 459–466. https://doi.org/10.1094/MPMI -12-23-0208-R
Zhao, P., Yang, H., Sun, Y., Zhang, J., Gao, K., Wu, J., Zhu, C., Yin, C., Chen, X., Liu, Q., Xia, Q., Li, Q., Xiao, H., Sun, H. X., Zhang, X., Yi, L., Zhou, C., Kliebenstein, D. J., Fang, R., Wang, X., & Ye, J. (2025). Targeted MYC2 stabilization confers citrus Huanglongbing resistance. Science, 388, 191–198. https://doi.org/10.1126/science.adq7203
Zheng, D., Armstrong, C. M., Yao, W., Wu, B., Luo, W., Powell, C., Hunter, W., Luo, F., Gabriel, D., & Duan, Y. (2024). Towards the completion of Koch's postulates for the citrus Huanglongbing bacterium, Candidatus Liberibacter asiaticus. Horticulture Research, 11(3), uhae011. https://doi.org/10.1093/hr/ uhae011
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Buitenzorg: Journal of Tropical Science

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
The article is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (CC BY-SA), which allows both Authors and Readers to copy and distribute the material in any format or medium, as well as modify and create derivative works from it for any purpose, provided that appropriate credit is given (by citing the article or content), a link to the license is provided, and it is indicated if any changes were made. If the material is modified or used to create derivative works, the contributions must be distributed under the same license as the original.






